Healgen Scientific Ltd
Corona Virus COVID-19 Antibody IgG/IgM Rapid Detection Kit 25/Box
- SKU:
- GCCOV-402a
- MPN:
- GCCOV-402a
Description
COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma)
Please call 800-915-7017 to place an order or get additional information. This product cannot be sold online and cannot be used for home use.
NOW EUA APPROVED (Emergency Use Authorization)
The COVID-19 IgG/IgM (Whole Blood/Serum/Plasma) Rapid Test Device utilizes lateral flow technology that is used for the qualitative, differential detection of both& anti-SARS-CoV-2 IgM and IgG antibodies. This test is intended to screen symptomatic patients for COVID-19. Results obtained should not be the sole determinant for clinical decision. Combining RNA and Antibody tests can significantly raise the sensitivity for detecting COVID-19 in infected individuals.
In general, antibodies can be detected 1-3 weeks after infection. This test is intended to screen patients for COVID-19. Combining RNA and Antibody tests can significantly raise the sensitivity for detecting COVID-19 in infected individuals.
Coronaviruses are enveloped RNA viruses that are distributed broadly among humans, other mammals ands birds that cause respiratory, enteric, hepatic and neurologic diseases. Four viruses - 229E, OC43, NL63 and HKU1 are prevalent and typically cause common cold symptoms in immunocompromised individuals. Three other strains SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) are can be transmitted from between non-human vertebrates to humans.
The Covid-19 virus is thought to spread mainly from person-to-person
- Between people who are in close contact with one another (within about 6 feet).
- Via respiratory droplets produced when an infected person coughs or sneezes.
- These droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs.
Features
- Detection Window (IgM): Symptomatic 3-5 days
- Dual band results for simple interpretation
- Multivariable analysis of immunoglobin IgG & IgM
- Room temperature storage or refrigerated (2-30⁰C / 36-86⁰F)
- Procedural internal control included
- Buffer included
- Fast results in 10 minutes
- Facilitates patient treatment decisions quickly
- Simple, time-saving procedure
- All necessary reagents provided & no equipment needed
- Little specimens, only 5 μL of serum/plasma or 10 μL of whole blood specimens
- High Sensitivity and Specificity
Instructions for Use
- Remove the test cassette from the sealed foil pouch and use it as soon as possible.
- Lay device on flat surface and provide specimen (see specific instructions for each specimen type below)
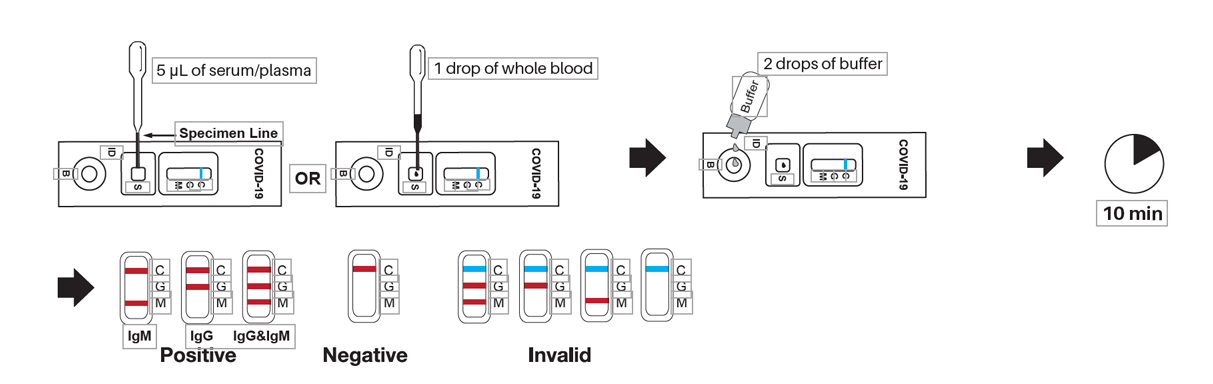
- For Serum or Plasma Specimen: with the 5μl plastic dropper provided, draw serum/plasma specimen to exceed the specimen line, as shown in the diagram below. Hold the dropper vertically and transfer drawn serum/plasma specimen into the sample well (S). Add 2 drops (about 80 μl) of sample buffer to the buffer well (B) immediately. Avoid air bubbles.
- For Whole Blood Specimen: Hold the 5 μl plastic dropper vertically and transfer 1 drop of whole blood (about 10 μl) to the specimen well (S) of the test device.Immediately add 2 drops (about 80 μl) of sample buffer to the buffer well (B). Avoid air bubbles.
- Wait for the colored line(s) to appear. If, after 2 minutes, the red color has not moved across the test window or if blood is still present in the specimen well (S) does not begin to appear test window, add 1 additional drop of sample buffer to the buffer well (B).
- The results should be read in 10 minutes. Do not interpret the result after 15 minutes.
FAQ
Q: Is the COVID-19 IgG/IgM Rapid Test Device FDA Approved?
A: The FDA has allowed for the distribution, sale and use of these kits in the United States prior to receiving FDA Emergency Use Authorization (EUA) for professional use with laboratories and healthcare workers at the point-of-care.
- This test has not beeen reviewed by the FDA
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic method should be considered to rule out infection in these individuals.
- Results from antibody testing should be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E
Q: How accurate is COVID-19 IgG/IgM Rapid Test Device?
A: A recent clinical evalutation of this test has demonstrated, when compared to a different method, a total agreement of 97.19% and a kappa value of 0.94. The overall study included 10 sites in China during February 2020, where 704 tests were used. Additional information is available upon request.
COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) Documentation |
||||
Specifications
- Sensitivity: IgG 97.2%; IgM 87.9%
- Specificity: IgG 100%; IgM 100%
- Specimen: Whole Blood, Serum, Plasma
- Time to Results: 10 minutes
- Shelf Life: 24 months from the date of manufacture
Sources: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html
Warning
- This test has been authorized by FDA under an EUA for use by authorized laboratories.
- This test has not been FDA cleared or approved.
- This test has been authorized only for the presence of IgM and IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens.
- This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
- This product is intended for professional use and not for home use.
- Not for the screening of donated blood.