FDA Employment & Insurance
The tests on this page are lateral flow, one-step immunoassay for the qualitative detection of specific drugs and their metabolites in human oral fluid for use in Employment and Insurance (E&I) testing.
Excerpt from Federal Register Website: The Food and Drug Administration (FDA or Agency) is announcing a list of class II devices that the Agency has determined based on established factors to no longer require premarket notification to provide reasonable assurance of safety and effectiveness, subject to certain limitations. FDA is publishing this notice of that determination in accordance with procedures established by the 21st Century Cures Act. The exemptions in this notice will decrease regulatory burdens on the medical device industry and will eliminate private costs and expenditures required to comply with certain Federal regulations. FDA is identifying the following list of class II devices that no longer require premarket notification under section 510(k) of the FD&C Act, subject to the general limitations to the exemptions found in §§ 862.9 to 892.9.
- Product
- Qty in Cart
- Quantity
- Price
- Subtotal
-

12 Panel SAFElife™ T-Square® Multi-Drug Oral Fluid Saliva Drug Test, 25/Box
Wondfo USA
$134.2512 Panel T-Square® Oral Saliva Drug Test Device with Saturation Indicator, 25/Box This T-Square® Saliva drug test is approved for Employment & Insurance Use (see panel configurations) T-Square® One Step Multi-Drug Oral Fluid Test Cube is...$134.25 -

10 Panel SAFElife™ T-Square® Multi-Drug Oral Fluid Saliva Drug Test, 25/Box
Wondfo USA
$124.2510 Panel T-Square® Oral Saliva Drug Test Device with Saturation Indicator, 25/Box This T-Square® Saliva drug test is approved for Employment & Insurance Use (see panel configurations) T-Square® One Step Multi-Drug Oral Fluid Test Cube is...$124.25 -

7 Panel SAFElife™ T-Square® Multi-Drug Oral Fluid Saliva Drug Test, 25/Box
Wondfo USA
$94.507 Panel T-Square® Oral Saliva Drug Test Device with Saturation Indicator, 25/Box This T-Square® Saliva drug test is approved for Employment & Insurance Use (see panel configurations) T-Square® One Step Multi-Drug Oral Fluid Test Cube is a...$94.50 -
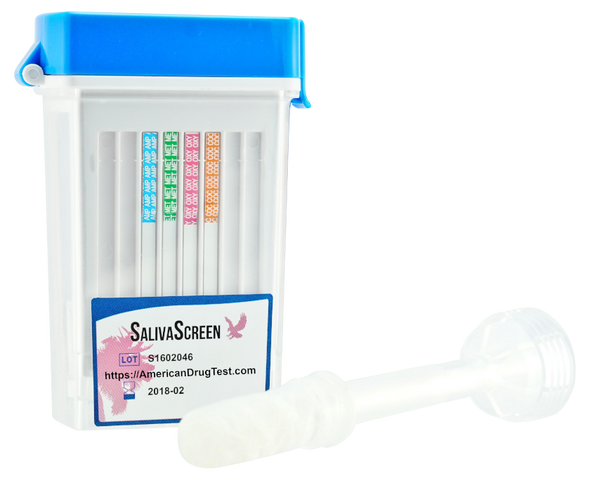

Accurate™ 10 Panel Oral Saliva Drug Test Flip Top Cube Device 25/Box
Healgen Scientific Ltd
$124.7510 Panel Oral Saliva Screen Drug Test Flip Top Cube Device American Drug Test is now selling the Healgen Scientific Accurate™ Rapid Saliva Drug test which is a cost effective way to detect illicit drugs within oral fluid. Designed for sensitivity,...$124.75 -

9 Panel SAFElife™ T-Square® Multi-Drug Oral Fluid Saliva Drug Test, 25/Box
Wondfo USA
$119.259 Panel T-Square® Oral Saliva Drug Test Device with Saturation Indicator, 25/Box This T-Square® Saliva drug test is approved for Employment & Insurance Use (see panel configurations) T-Square® One Step Multi-Drug Oral Fluid Test Cube is a...$119.25 -

5 Panel SAFElife™ T-Square® Multi-Drug Oral Fluid Saliva Drug Test, 25/Box
Wondfo USA
$92.005 Panel T-Square® Oral Saliva Drug Test Device with Saturation Indicator, 25/Box This T-Square® Saliva drug test is approved for Employment & Insurance Use (see panel configurations) T-Square® One Step Multi-Drug Oral Fluid Test Cube is a...$92.00 -

6 Panel SAFElife™ T-Square® Multi-Drug Oral Fluid Saliva Drug Test, 25/Box
Wondfo USA
$100.006 Panel T-Square® Oral Saliva Drug Test Device 25/Box This T-Square Saliva drug test is approved for Employment & Insurance Use (see panel configurations) T-Square® One Step Multi-Drug Oral Fluid Test Cube is a rapid oral fluid screening test...$100.00 -
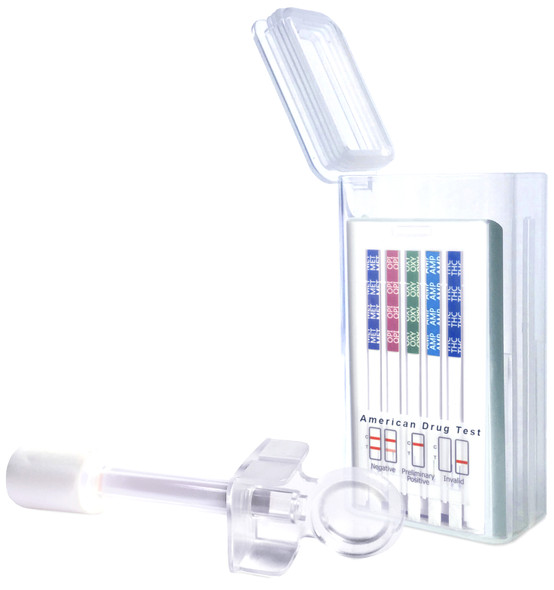
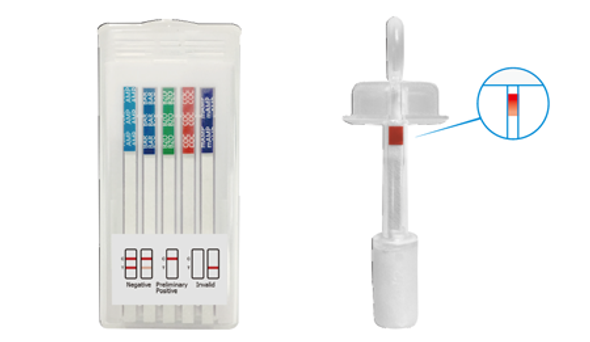
5 Panel T-Cube® One Step Multi-Drug Oral Fluid Saliva Drug Test 25/Box
Abbott Diagnostics
$198.005 Panel T-Cube Oral Saliva Drug Test Device 25/Box New Item # ABTOFCUBE0501A fomerly #T-Cube-5CO T-Cube Saliva drug test is approved for Employment & Insurance Use (see below) T-Cube® One Step Multi-Drug Oral Fluid Test Cube is a rapid oral fluid...$198.00 -


7 Panel T-Cube® Oral Fluid Drug Test For Employment & Insurance Testing
Abbott Diagnostics
$208.507 Panel T-Cube Oral Saliva Drug Test Device from Abbott Diagnostics, 25/Box New Item # ABTOFCUBE0701A The T-Cube Saliva drug test is approved for Employment & Insurance Use T-Cube® One Step Multi-Drug Oral Fluid Mouth Swab Test Cube is a rapid...$208.50 -


10 Panel T-Cube® Oral Fluid Drug Test For Employment & Insurance Testing
Abbott Diagnostics
$234.0010 Panel T-Cube Oral Saliva Drug Test Device 25/Box New Item # ABTOFCUBE1001A The T-Cube Saliva drug test is approved for Employment & Insurance Use T-Cube® One Step Multi-Drug Oral Fluid Test Cube is a rapid oral fluid screening test...$234.00 -


6 Panel T-Cube® Oral Fluid Drug Test For Employment & Insurance Testing
Abbott Diagnostics
$202.006 Panel T-Cube Oral Saliva Drug Test Device 25/Box New Item # ABTOFCUBE0602B The T-Cube Saliva drug test is approved for Employment & Insurance Use T-Cube® One Step Multi-Drug Oral Fluid Test Cube is a rapid oral fluid screening test. The test is...$202.00 -


9 Panel T-Cube® Oral Fluid Drug Test For Employment & Insurance Testing 25/Box
Abbott Diagnostics
$220.009 Panel T-Cube Oral Saliva Drug Test Device 25/Box The T-Cube Saliva drug test is approved for Employment & Insurance Use T-Cube® One Step Multi-Drug Oral Fluid Test Cube is a rapid oral fluid screening test. The test is a lateral flow,...$220.00
