Healgen Scientific Ltd
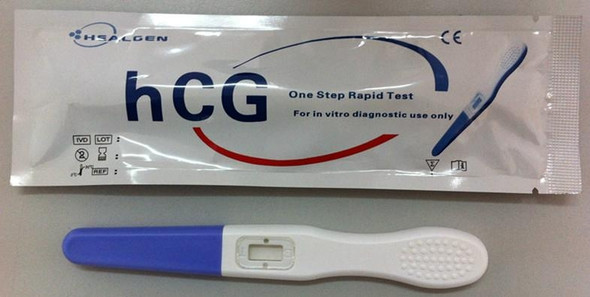
One Step hCG Pregnancy Test Strip FDA Cleared, CLIA Waived & OTC 50/BX
- SKU:
- GAHCG-101a
- MPN:
- GAHCG-101a
Bulk discount rates
Below are the available bulk discount rates for each individual item when you purchase a certain amount
| Quantity | Per Item |
| 1 - 3 | $10.25 |
| 4 - 9 | $9.75 |
| 10 - 19 | $9.25 |
| 20+ | $8.75 |
Description
One Step hCG Pregnancy Test Strip CLIA Waived 50/Box
The hCG One Step Pregnancy Test (Urine) is a rapid chromatographic immunoassay for the qualitative detection of human chorionic gonadotropin (hCG) in urine to aid in the early detection of pregnancy.
Human chorionic gonadotropin (hCG) is a hormone produced by the developing placenta shortly after conception and secreted into the urine and serum. hCG can be detected in both urine and serum as early as 7 to 10 days after conception. hCG levels continue to rise very rapidly, frequently exceeding 100 mIU/mL by the first missed menstrual period, and peaking in the 100,000 - 200,000 mIU/mL range about 10 - 12 weeks into pregnancy. One Step hCG Pregnancy Test Strip FDA Cleared CLIA Waived & OTC 50/BX is an accurate and economical choice for medical facilities and clinics requiring rapid pregnancy tests.
Human chorionic gonadotropin (hCG) is a glycoprotein hormone produced in pregnancy that is made by the developing embryo after conception and later by the syncytiotrophoblast. In a normal pregnancy, hCG can be detected in urine as early as 1-2 weeks after conception. hCG levels continue to rise very rapidly, usually exceeding 100 mIU/mL by the first missed menstrual period, and peaking in the 100,000-200,000 mIU/mL range about 10-12 weeks into pregnancy. Early pregnancy testing, in general, is based on the detection or measurement of hCG. The Pregnancy One Step Rapid Test utilizes a combination of monoclonal and polyclonal antibodies to selectively detect elevated levels of hCG in urine. Positive specimens react with the specific antibody-hCG-colored conjugate to form a colored line at the test line region of the membrane. Absence of this colored line suggests a negative result. At the level of claimed sensitivity, the Pregnancy One Step Rapid Test shows no cross-reactivity interference from the structurally related glycoprotein hormones hFSH, hLH, and hTSH at high physiological levels.
Features
- FDA Clia Waived 510(k) # K123703
- CE Marked
- FDA Cleared
- OTC (Over-The-Counter) Non-prescription approved by the FDA
- Test Results in 5 Minutes
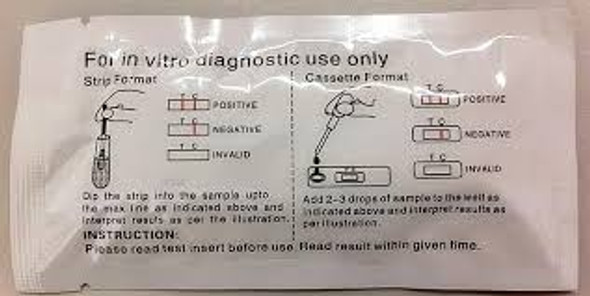
- Cassette Format with Pipette
- 25 mIU/ml Sensitivity
- One Step Rapid Test (Cassette) is an in vitro diagnostic visual qualitative immunochromatographic assay
- Rapid test for the determination of human chorionic gonadotropin (hCG) in urine
- Reliable & Easy to Use
- Easy to operate and simple interpretation
- Room temperature storage or refrigerated (2-30⁰C)
- Internal control included
Additional
- Private Label Options Available
- Strip and Midstream Formats Available
- 25/Box or 50/Box Options
Specifications
- Cut-off(s): See ordering information
- Specimen: Urine
- Time to Results: 5 minutes
- Shelf Life: 24 months from the date of manufacture
| hCG Format Options | |||||
| Product Name | Specimen | Format | Sensitivity | Catalog No. | Approvals & Certifications |
| hCG Pregnancy Rapid Test | Urine | Strip | 25 mIU/mL | GAHCG-101a | FDA Cleared CLIA Waived CE Marked |
| hCG Pregnancy Rapid Test | Urine | Cassette | 25 mIU/mL | GAHCG-102a | FDA Cleared CLIA Waived CE Marked |
| hCG Pregnancy Rapid Test | Urine | Midstream | 25 mIU/mL | GAHCG-103a | FDA Cleared CLIA Waived CE Marked |
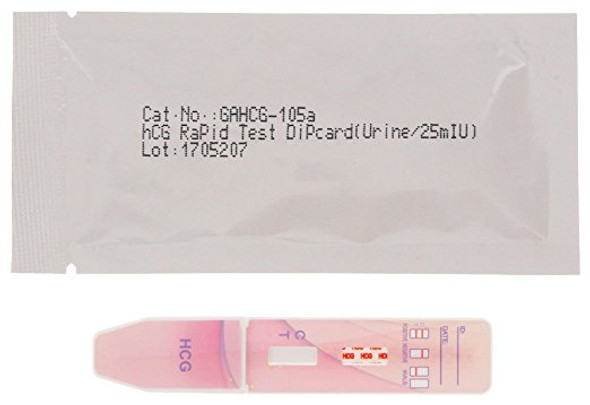
| hCG Pregnancy Rapid Test | Urine | Dip Card | 25 mIU/mL | GAHCG-105a | FDA Cleared CLIA Waived CE Marked |