Alfa Scientific
Instant-View hCG Pregnancy Combo Serum / Urine Test Cassette CLIA Waived, OTC, FDA Approved 25/Box
- SKU:
- 02-2483
- MPN:
- 02-2483
Description
Instant-view® hCG Rapid Pregnancy Test Serum / Urine Cassette from Alfa Scientific Designs, Inc.
The Instant-View Pregnancy Combo (Urine/Serum) Cassette, is a qualitative immunoassay for the detection of human chorionic gonadotropin (hCG) in human urine or serum for the early detection of pregnancy. A more specific alternate chemical method must be used in order to obtain a confirmed analytical result. FDA Cleared and CLIA Waived for urine. Moderately Complex for use with serum. (FDA 510K#: K984080)
Technology: Instant-view® Lateral Flow Test
Specimen: Urine, Urine/Serum
Format: Cassette
Sensitivity: 25 mIU/ml
Features
- Developed & Manufactured in the USA
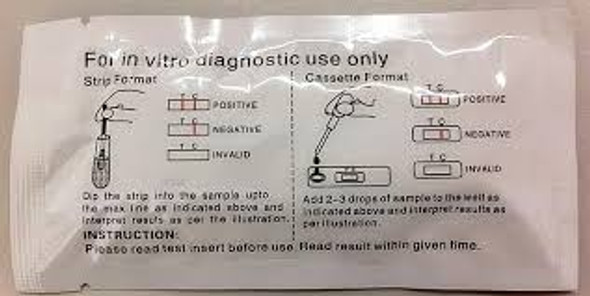
- The Pregnancy One Step Rapid Test (Midstream) is an in vitro diagnostic visual qualitative immunochromatographic assay designed for the rapid determination of human chorionic gonadotropin (hCG) in urine to aid in the detection of pregnancy
- The test is for self testing
- FDA Cleared for professional/CLIA/OTC market
- OTC (Over-The-Counter) Non-prescription approved by the FDA
- CE Marked
- Sensitivity 25 mIU / mL
| Product Specifications | |
| Manufacturer # | 02-2483 |
| Manufacturer | Alfa Scientific |
| Application | Rapid Diagnostic Test |
| CLIA Classification | CLIA Waived |
| Kit Contents | Cassette Test Device, Dropper Pipette, Instructions for Use |
| Number of Tests | 25 Test Cassettes |
| Reading Type | Visual Read |
| Sample Type | Serum / Urine Sample |
| Test Format | Cassette with Pipette |
| Test Name | hCG Pregnancy Test |
| Test Type | One-Step |
| Time to Results | 5 Minute Results |
| UNSPSC Code | 41116205 |
Human chorionic gonadotropin (hCG) is a glycoprotein hormone produced in pregnancy that is made by the developing embryo after conception and later by the syncytiotrophoblast. In normal pregnancy, hCG can be detected in urine as early as 1-2 weeks after conception. hCG levels continue to rise very rapidly, usually exceeding 100 mIU/mL by the first missed menstrual period, and peaking in the 100,000-200,000 mIU/mL range about 10-12 weeks into pregnancy. Early pregnancy testing, in general, is based on the detection or measurement of hCG. The Pregnancy One Step Rapid Test utilizes a combination of monoclonal and polyclonal antibodies to selectively detect elevated levels of hCG in urine. Positive specimens react with the specific antibody-hCG-colored conjugate to form a colored line at the test line region of the membrane. Absence of this colored line suggests a negative result. At the level of claimed sensitivity, the Pregnancy One Step Rapid Test shows no cross-reactivity interference from the structurally related glycoprotein hormones hFSH, hLH and hTSH at high physiological levels.