AllTest Biotech Co, Ltd
AllTest® 18 Panel Rapid Drug Test Cup, CLIA Waived with EDDP, FEN, TRA, 6-MAM and 3 Adulterants, 25/Box
Free FedEx Ground shipping on orders of $150 or more
Sales Tax Exempt? Call 800-915-7017 before ordering
- SKU:
- DUA-A1187C-002
- Availability:
- In stock and ready to ship from our California facility. Orders placed by 2:00 PM ET ship the same business day.
Bulk discount rates
Below are the available bulk discount rates for each individual item when you purchase a certain amount
| Quantity | Per Item |
| 1 - 3 | $158.75 |
| 4+ | $152.40 |
Description
AllTest® 18 Panel Multi-Drug Rapid Test Cup with CLIA Waived, Fentanyl, Tramadol, EDDP, 6-MAM, and 3 Adulterants, 25/Box
The AllTest 18 Panel Rapid Drug Test Cup CLIA Waived delivers comprehensive screening in 5 minutes. Built-in adulterants ensure sample integrity; American Drug Test supplies 25-box packs.
Features
- Rapid test for the simultaneous, qualitative detection of multiple drugs and drug metabolites in human urine
- 6MAM-10, AMP500, BAR300, BUP10, BZO300, COC150, EDDP300, FYL1, MDMA500, MET500, MOP(OPI)300, MTD300, OXY100, PCP25, PPX300, TCA1000, THC50, TRA(TML)100 + OX CR PH
- CLIA Waived
- The test utilizes monoclonal antibodies to selectively detect elevated levels of specific drugs in the specimen
- Fast results in 5 minutes
- Easy visual interpretation and high accuracy
- Simple operation, no equipment required
- For healthcare professionals, including professionals at point-of-care sites
Download Product Documentation
![]() AllTest Multi-Drug CLIA Waived Rapid Drug Test Cup Package Insert
AllTest Multi-Drug CLIA Waived Rapid Drug Test Cup Package Insert
Directions for Use
Allow the test, urine specimen, and/or controls to reach room temperature 59–86°F (15–30ºC) prior to testing.
- Bring the pouch to room temperature before opening it. Remove the cup from the sealed pouch.
- The donor provides the specimen.
- The technician replaces and secures the cap while the cup is on a flat surface.
- Check the temperature label (Temp Label) up to 4 minutes after specimen collection. A green color will appear to indicate the temperature of the urine specimen. The proper range for an unadulterated specimen is 90–100°F (32–38°C).
- The technician dates and initials the security seal and attaches the security seal over the cup cap.
- The technician peels off the label to reveal adulteration strip(s), if applicable.
- The technician peels off the label on the multi-drug test cup to view results.
- The adulteration strip(s), if applicable, should be read between 3–5 minutes. Compare the colors on the adulteration strip to the color chart. If the results indicate adulteration, do not read the drug test results. Refer to your Drug-Free Policy for guidelines on adulterated specimens. We recommend not interpreting the drug test results and either retesting the urine or collecting another specimen in case of any positive result for any adulteration test.
- If results do not indicate adulteration, read the drug test result at 5 minutes. Do not interpret the result after 10 minutes.
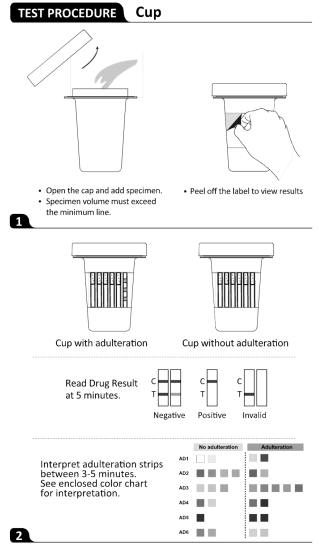
Interpretation of Results
(Please refer to the illustration above)
Negative
A colored line appears in the Control region (C), and a colored line appears in the Test region (T). This negative result means that the concentrations in the urine sample are below the designated cut-off levels for a particular drug tested.
Note: The shade of the colored line(s) in the Test region (T) may vary. The result should be considered negative whenever there is even a faint line.
Positive
A colored line appears in the Control region (C, and no line appears in the Test region (T). The positive result means that the drug concentration in the urine sample is greater than the designated cut-off for a specific drug.
Invalid
No line appears in the Control region (C). Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for Control line failure. Read the directions again and repeat the test with a new test cup. If the result is still invalid, contact your manufacturer.
Interpretation of Results (S.V.T. Adulteration)
Semi-quantitative results are obtained by visually comparing the reacted color blocks on the strip to the printed color blocks on the color chart. No instrumentation is required. Please refer to the color chart.